Registration for this event has been disabled.
Global | Innovative | Unbiased | Comprehensive | Multidisciplinary | Insightful
GLOBAL | Regulatory agencies, the health care industry, academia, and patient organizations worldwide rely on DIA’s unique global community for access to knowledge resources and opportunities for networking and professional development. With reach into established and emerging markets, DIA provides a balanced forum for exchanging knowledge, establishing relationships, and advancing careers.
INNOVATIVE | DIA raises the level of discussion to facilitate innovation that will change the future of health care. DIA’s relationships with the full spectrum of health care industry stakeholders, academics, and regulators fosters the discovery, development, and life cycle management of new medicinal products that are safer, more effective, and affordable, and reach the market faster to increase global access and advance regulatory science.
UNBIASED | DIA has five decades of experience as an unbiased convening body that provides an open scientific forum for discussing critical issues, challenges, and opportunities related to health care innovation; the discovery, development, and life cycle management of medicinal products; increasing access to medicinal products; and improving health care worldwide.
COMPREHENSIVE | DIA’s relationships, resources, and knowledge encompass the full range of the life cycle management process for the entire universe of medicinal products, including pharmaceuticals, biotechnology, and medical devices.
MULTIDISCIPLINARY | DIA approaches the discovery, development, and life cycle management of regulated medical products in a holistic and integrated manner.DIA-sponsored programs allow organizations to bring the full spectrum of professionals together to discuss best practices and to develop enterprise-wide strategies.
INSIGHTFUL | DIA is a leader in advancing the conversation about the discovery, development, and life cycle management of regulated medical products.
DIA is the first organization that thought leaders, health care professionals, regulatory officials, educators, and patient organizations turn to for timely and authoritative information. The conversation begins with DIA—through knowledge-sharing programs such as conferences, training courses, online learning, networking events, and publications.
INNOVATIVE | DIA raises the level of discussion to facilitate innovation that will change the future of health care. DIA’s relationships with the full spectrum of health care industry stakeholders, academics, and regulators fosters the discovery, development, and life cycle management of new medicinal products that are safer, more effective, and affordable, and reach the market faster to increase global access and advance regulatory science.
UNBIASED | DIA has five decades of experience as an unbiased convening body that provides an open scientific forum for discussing critical issues, challenges, and opportunities related to health care innovation; the discovery, development, and life cycle management of medicinal products; increasing access to medicinal products; and improving health care worldwide.
COMPREHENSIVE | DIA’s relationships, resources, and knowledge encompass the full range of the life cycle management process for the entire universe of medicinal products, including pharmaceuticals, biotechnology, and medical devices.
MULTIDISCIPLINARY | DIA approaches the discovery, development, and life cycle management of regulated medical products in a holistic and integrated manner.DIA-sponsored programs allow organizations to bring the full spectrum of professionals together to discuss best practices and to develop enterprise-wide strategies.
INSIGHTFUL | DIA is a leader in advancing the conversation about the discovery, development, and life cycle management of regulated medical products.
DIA is the first organization that thought leaders, health care professionals, regulatory officials, educators, and patient organizations turn to for timely and authoritative information. The conversation begins with DIA—through knowledge-sharing programs such as conferences, training courses, online learning, networking events, and publications.
Click Here to Join DIA, go with us.
Membership In China
The Advisory Council of China (ACC) includes regional industry and academic thought leaders, regulators and researchers who are responsible for creating a sense of community among those who support the DIA vision to provide a global forum for knowledge exchange that fosters innovation to raise the level of health and well-being worldwide.
Join the world’s premier member association dedicated to the discovery, development, and life cycle management of pharmaceuticals, biotechnology, medical devices, and related products.
DIA’s 18,000 members are professionals and experts from around the globe working to advance innovation in the pharmaceutical, biotechnology, medical device, and related fields. Professionals worldwide look to DIA as the only global multidisciplinary association that provides a neutral forum to address the challenges you face every day. Take advantage of all the benefits of membership to network, learn, and lead.
Join the world’s premier member association dedicated to the discovery, development, and life cycle management of pharmaceuticals, biotechnology, medical devices, and related products.
DIA’s 18,000 members are professionals and experts from around the globe working to advance innovation in the pharmaceutical, biotechnology, medical device, and related fields. Professionals worldwide look to DIA as the only global multidisciplinary association that provides a neutral forum to address the challenges you face every day. Take advantage of all the benefits of membership to network, learn, and lead.
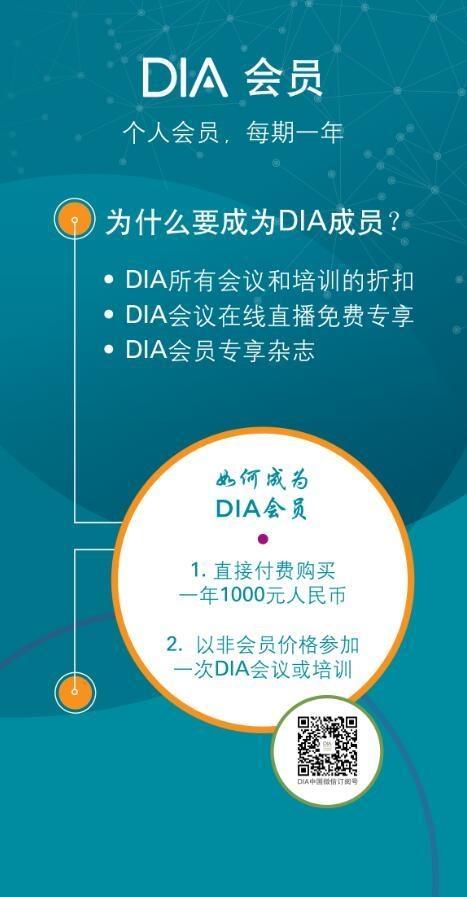

DIA Membership Benifits
- Access to DIA ConneX Membership Directory - online networking platform to create your own personal network of colleagues and thought leaders globally.
- Subscriptions to:
- Drug Information Journal, DIA’s peer reviewed scholarly journal (6 issues)
- DIA Global Forum, DIA’s newsmagazine featuring association news & industry articles (6 issues)
- Access to searchable index of DIA articles
- Member discounts to DIA’s local events including the Latin America Congress on Clinical Research as well as over 200 educational offerings worldwide
- Special access to our comprehensive online career center
- Career development and networking opportunities as a member of Special Interest Area Communities (SIACs), global network of member subgroups
- Opportunities to join committees and to volunteer as an author, session chair, or speaker
- Networking opportunities with DIA ConneX – our state-of-the-art members-only platform that allows you to connect with professional colleagues anywhere in the world and build trusted networks where interactive global communication and information exchange can occur.
- eNewsletters and DIA Daily: timely regulatory updates delivered to your inbox
- Contract Service Organization Directory
DIA Publication


Global Forum
The Global Forum includes:
- See more at: http://www.diahome.org/zh-CN/News-and-Publications/Publications-and-Research/GF.aspx#sthash.ozA8BIpu.dpuf
The Global Forum includes:
- Important news from DIA conferences and workshops
- DIA Board of Director and Regional Advisory Council reports—news that directly impacts DIA members
- Practical tips, regulatory and global updates, upcoming DIA events, program notes, and much more
- See more at: http://www.diahome.org/zh-CN/News-and-Publications/Publications-and-Research/GF.aspx#sthash.ozA8BIpu.dpuf
Therapeutic Innovation & Regulatory Science
This newly launched Journal rebrands the Drug Information Journal, which DIA proudly published for 46 years. The scope of Therapeutic Innovation & Regulatory Science reaches beyond pharmaceutical research and development to include innovations in drugs, devices, and diagnostics, as well as global regulatory issues.
- See more at: http://www.diahome.org/zh-CN/News-and-Publications/Publications-and-Research/TIRS.aspx#sthash.iVNGcEUB.dpuf
This newly launched Journal rebrands the Drug Information Journal, which DIA proudly published for 46 years. The scope of Therapeutic Innovation & Regulatory Science reaches beyond pharmaceutical research and development to include innovations in drugs, devices, and diagnostics, as well as global regulatory issues.
- See more at: http://www.diahome.org/zh-CN/News-and-Publications/Publications-and-Research/TIRS.aspx#sthash.iVNGcEUB.dpuf
Contact Us
DIA China
tel. 010-57042659
email. china@diaglobal.org
tel. 010-57042659
email. china@diaglobal.org